A. HYDROGEOLOGIC CHARACTERIZATION
REMOTE SENSING TECHNOLOGIES
Remote sensing can be simply defined as an observation from a distance, as opposed to in situ testing in which measuring devices are either immersed in or touch the object of observation and measurement. The most important physical links between objects of measurement and remote sensing measuring devices are electromagnetic energy, acoustic waves, and force fields. This brief review of remote sensing technologies focuses on electromagnetic energy as the medium for remote sensing. The application of remote sensing systems to the study of subsurface pollution involves a number of steps: recognition, identification, recording of distribution, assessment of distribution, and the understanding of the nature and cause of subsurface contamination. Because of the lack of immersion and direct contact between remote sensors and the measured objects, remote sensing is typically used in reconnaissance, and preliminary studies of subsurface pollution. Regional and local-scale geologic, cultural and natural surface features can be mapped rapidly to assess the likely presence of subsurface pollution and its spatial extent. Based on the type of sensors that are available for remote sensing, remote sensing technologies can be classified into passive sensing and active sensing systems. Each of these two broad classes of systems, passive and active, are in turn further subdivided according to the range of wavelengths of the electromagnetic spectrum to which they are applied. The following is a brief summary of remote sensing technologies. In depth coverage can be found in Barrett and Curtis (1992), or Drury (1993) which reviews remote sensing applications earth resources studies.
Passive Sensors
Passive sensors receive natural electromagnetic emissions that are then used to construct an image of the targeted environment. Passive sensors are divided into two broad categories: photographic and non-photographic systems. Photographic systems operate in the visible and near infrared parts of the electromagnetic spectrum (0.3 - 0.9 m m) only, whereas non-photographic sensors can range from X-ray to radio wavelengths. Examples of passive sensors which operate in the visible wavelengths are photographic cameras (airborne or mounted in space platforms) and vidicon cameras. The photographic camera has played an important role in the analysis of surface and structural geologic features that have proven useful in the analysis of subsurface contamination. The photographic camera uses a photographic emulsion to record the image of the targeted environment on a flexible film base. The principal advantages of the photographic camera are its large information storage capacity, high ground resolution, relatively high sensitivity, and high reliability. The main shortcomings of aerial photography are that exposures can be made only in daylight and cloud obscures ground detail. Examples of passive sensors outside the visible wavelengths are infrared sensors (radiometer and spectrometers), microwave radiometers, and absorption spectrometers.
Active Sensors
Active remote sensing systems use electromagnetic wave generators placed near the sensor which aim and propagate waves toward the earth surface where they are bounced off and recorded by the sensor upon their return. The recorded signal is processed to extract information and map the targeted surface or medium. Examples of active sensors are the radar (radio direction and ranging system), the microwave radar, and the lidar (or laser radar). The synthetic aperture radar (SAR), the real aperture radar (RAR), and the scatterometer are specific instruments based on the radar. Unlike the microwave radar, the lidar operates in that part of the electromagnetic spectrum comprising the ultraviolet to near infrared wavelengths. It consists of a laser which emits radiation in pulse or continuous mode through a collimating system. A second optical system collects the radiation returned and focuses it on to a detector. Three types of lidar are available at present: an altimeter type, which can plot a terrain profile, a scanning type, which can be used as a surface mapping instrument, and a third type using spectroscopic techniques, which can be used for mapping air constituents. The ground penetrating radar is an example of an active remote sensing system, which will be reviewed later in the section dealing with geophysical technologies.
SURFACE GEOPHYSICS TECHNOLOGIES
Geophysical techniques are used to determine indirectly the extent and nature of the geologic materials beneath the surface. Features that can be determined with some degree of approximation include the thickness and lateral extent of unconsolidated deposits, the depth to water table, changes in the salinity of groundwater, the location of geologic faults, depth to bedrock, lithological differentiation, etc. The correlation of geophysical data with well logs or test boring data is generally more reliable than either type of information used by itself. A thorough review of this subject is found in Zohdy, Eaton, and Mabey (1974). Fetter (1993, 1994) also presents good summaries of geophysical techniques.
Electrical Resistivity
A commutated direct current or an alternating current of very low frequency (less than 1 cycle per second) is generated in the field or provided by storage batteries. The current is introduced into the ground by means of two metal electrodes. The voltage in the ground is measured between two other metal electrodes, also driven into the ground. There are several electrode configurations in common usage: the Wenner, the Schlumberger, and the dipole-dipole array. The resistivity of the earth materials located between the current generating electrodes is determined based on the measured voltage and the electrical current flowing through the ground. The electrical resistivity, r , is given by r = (A/L) (V/I), in which A is the cross-sectional of current flow, L is the length of the current flowpath, V is the voltage, and I is the electrical current. r is expressed in ohm× m. Electrical conductivity is the inverse of resistivity, and it is expressed in ohm-1/m or, equivalently, in mho/m.
Electromagnetic Conductivity
Electromagnetic conductivity is the inverse of electrical resistivity. Electromagnetic methods use an electromagnetic field generated by a transmitter coil through which an alternating current is passed. This generates a magnetic field around the transmitter coil. When the transmitter coil is held near the earth, the magnetic field induces an electrical field in the earth. The strength of the electrical field established in the ground depends on its conductivity. The electrical field strength is measured in a passive receiver coil. Changes in the phase, amplitude, and orientation of the electrical field are measured as a function of either time or distance by the passive receiver coil. These changes are related to the electrical properties of the earth, from which the electromagnetic conductivity can then be estimated. There are several electromagnetic methods available. They all have the advantage of being rapid, as none of them require the insertion of electrodes into the ground. Electromagnetic methods are not inherently more accurate than electric resistivity methods but they are typically more cost-effective, due to simpler field work. Electromagnetic methods have been used to detect changes in earth conductivity related to contaminant plumes, buried metallic objects (e.g., tanks, drums), and sea-water/fresh water interfaces.
Seismic Methods
These methods create artificial seismic waves that travel through the ground. The seismic wave generated at the energy source travels in the subsurface and is detected by geophones located a specified distance from the source. In the seismic refraction method, widely used in hydrogeologic characterization, the nature of subsurface layering is discerned from the analysis of the arrival time vs. distance data captured by the geophones. The energy source that generates the seismic waves is a small explosive charge detonated in a shallow drill hole. One or two sticks of dynamite are sufficient for layered systems in which bedrock depth is on the order of 50 m. For shallower work (less than 15m), a sledgehammer struck on a steel plate lying on the ground may be a sufficient energy source. Typical applications of seismic refraction include the determination of depth to bedrock, slope of the bedrock, depth to water table, and in some cases, general lithology.
Ground Penetrating Radar (GPR)
This technology uses a source of electromagnetic radiation in the frequency range of 10 to 1,000 MHz. The radiation is applied at the ground surface, from which it travels to greater depths. The electromagnetic wave pulses are reflected back to the surface as they encounter an interface between two materials with different dielectric properties. The interfaces that cause the reflections may be due to such things as a change in lithology, groundwater salinity, the presence of a water table, or the presence of buried objects (e.g., underground tanks). The reflected wave pulse in detected by a receiving antenna in the GPR. The reflected pulse is recorded on a magnetic tape and displayed graphically. The GPR unit is pulled on the ground in a pattern of parallel lines, thus producing a continuous profile of the subsurface in the study area.
The depth of penetration of the GPR signal is a function of the type of geologic material and the frequency of the electromagnetic wave. Lower frequency waves penetrate a given medium to greater depths while higher frequencies do not penetrate as deeply but give greater resolution of subsurface interfaces. Wave penetration in fine-grained glaciolacustrine sediments has been found to be on the order of 10 m while that in coarse sand and gravel has been documented to reach 100 m. Dense clays deposits greatly inhibit GPR signal penetration.
Magnetometer Surveys
These measure the strength of the earth's magnetic field. A proton nuclear magnetic resonance magnetometer is frequently used. This is a hand-held instrument and one person can rapidly perform a survey over a site a few acres in size. A grid system is set up and measurements are made of the magnetic field at each intersection of the grid. Areas with large amounts of buried metal, such as metal steel or iron metal drums, will have magnetic anomalies associated with them. The strength of the anomaly varies with the amount and depth of buried objects.
BOREHOLE GEOPHYSICS TECHNOLOGIES
Borehole geophysical technologies use probes lowered into a borehole on a cable. The probe contains the necessary electronic, electromagnetic, nuclear, video, acoustic, or physical devices that gathered information as the probe is lowered or raised inside the borehole. The type of data that can be gathered with borehole geophysical technologies is large, varying from physical properties of soil and rocks to the dispersion, dilution, and movement of waste (see, e.g., Keys and MacCary, 1971; Fetter, 1994). Only a few technologies are reviewed herein, as we focused on those that may have greater applicability in environmental monitoring and site characterization at DOE sites. The nuclear logging technologies of the next section are borehole geophysical technologies as well.
Resistivity Surveys
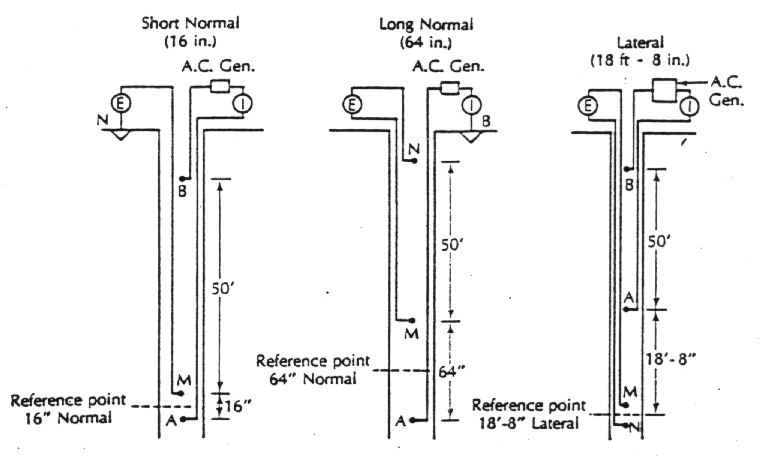
Resistivity, r , is defined by r = (A/L) (V/I), where A is the cross section of porous medium through which electrical current flows, L is the distance between electrodes, V is the applied voltage, and I is the electrical current established between electrodes. r is expressed in ohm× m. Earth (electric) resistivity is measured in a borehole by lowering two current electrodes and measuring the resistivity between two additional electrodes under an applied voltage and electrical current. The electric resistivity measures the ability of a porous medium and its pore water to transmit electricity. When correlated with hydraulic conductivity, resistivity surveys have the potential for cost-effective aquifer characterization. The figure below shows three different electrode configurations used in resistivity logging. The short normal configuration measures the resistivity of the zone close to the borehole. The long normal spacing has more spacing between electrodes and this measure the resistivity farther away form the borehole. Lateral configurations have very widely spaced electrodes for measuring zones that are far from the borehole. Because of the wide spacing, lateral devices will not distinguish among thin beds of different resistivity.

Cross-Borehole Tomography
Cross-borehole tomography resembles the surface geophysical methods in the sense that there are sources and receptors of electrical, seismic, or electro-magnetic signals, but in cross-borehole tomography the sources and receptors are located inside different boreholes. This allows a mapping of subsurface characteristics both as a function of depth and of lateral separation between the source and receptor boreholes.
NUCLEAR LOGGING TECHNOLOGIES
Nuclear logging technologies can be applied in shallow or deep boreholes, depending on the target medium to be characterized. These technologies measure either the radioactivity of rocks and fluids or their attenuation of induced radiation. Nuclear logging in the former mode measures the number of radioactive atomic disintegrations over a fixed time interval called the time constant. The longer the time constant, the lower the probability that a variation in radiation intensity is due to random decay and, thus, the more likely the variation reflects differences in lithology and pore-fluid composition. Important considerations in the performance of nuclear logging are the logging speed and the selection of the time constant.
Natural Gamma Radiation
This method measures the natural radiation of rocks and pore fluids as determined by the emission of gamma radiation by potassium 40, the uranium 238 decay series, and the thorium 232 decay series. No radiation source is needed in a natural gamma logging and it can be performed in a cased borehole.
Neutron Logging
A neutron probe contains a radioactive element, such as PbBe, which serves as a source of neutrons, and a detector. The emitted neutrons at the source are slowed and scattered by collisions with nuclei of hydrogen atoms. A detector is available to measure gamma radiation produced by the neutron-hydrogen atom collisions, or by the number of neutrons present at different energy levels. Therefore, a neutron log will be identified as a neutron-thermal neutron, a neutron-epithermal neutron, or a neutron-gamma log on the basis of the method of detection. Hydrogen atoms are present in the subsurface primarily in the form of water or hydrocarbons. When water (and thus hydrogen) is present it is responsible, apart from hydrated minerals, for the larger moderation or capture of neutrons emitted by the neutron source. Saturated porous media of high porosity have lower neutron counts than low-porosity rocks. Above the water table, neutron logging equipment can measure the moisture content, as well as it can detect leachate leaks under waste disposal sites. Hydrocarbon contaminants in unsaturated soils can also be detected by neutron logging due to their hydrogen atom content and ability to capture emitted neutrons.
Gamma-Gamma Radiation
A source of gamma radiation, such as 60Co is lowered within the borehole. Gamma photons are absorbed or scattered by all materials with which the 60Co comes in contact. The absorption depends on the bulk density of the earth material surrounding the gamma source. The bulk density is defined as the mass of rock or soil divided by its total volume. The gamma-gamma radiation increases with the decreasing bulk density (increasing porosity). Bulk density (and thus, porosity) can be estimated from a calibrated gamma-gamma log. The calibration of a gamma-gamma log requires the independent determination of bulk density, which can then be compared with the gamma-gamma log in search for a relationship between gamma-gamma measurements and bulk density. Once bulk density, r b, is known, the porosity, n, is obtained from the relationship, n = 100 (1 - r b/r p), in which r p is the particle density.
DRILLING TECHNOLOGIES
An excellent summary of well drilling methods is found in Driscoll (1986). All of these methods are applicable to monitoring wells for groundwater and soil/rock sample collections. With proper precautions and standard operating procedures, the drilling methods reviewed below are applicable to well installation and sample collection for environmental monitoring purposes (Fetter, 1993).
Geoprobe and SCAP Systems
The Geoprobe system consists of a string of coupled rods that have a drive point attached at their end. The drive point provides penetration capability into the ground. The rods and drive point are pushed down into the ground by a hydraulic system mounted on a truck. The rods, drive point, and hydraulic push system constitute a so-called penetrometer system. The hydraulic system transmits part of the truck's weight to the penetrometer to drive it through unconsolidated deposits. It also provides a hydraulic hammering capability to break down small rocks encountered along the penetrometer's path. Soils samples can be obtained at any desired depth not exceeding 20 m (beyond this depth, buckling and pull-back friction compromise the effectiveness of the penetrometer system). Groundwater samples can also be obtained at any depth (not exceeding 20 m) by attaching a prepacked, screened, small diameter pipe between the end of the rods and the drive point. A bailer is introduced through the hollow rods to retrieve groundwater samples. A suite of probes can be attached at the end of the penetrometer system, such as electrical conductivity probes and other borehole geophysical sensors, giving rise to a site characterization and analysis penetrometer system (SCAP system, or SCAPS). The Geoprobe penetrometer is fast, reliable, cost-effective, and introduces minimum disturbance in the subsurface. It is limited to soft soils and depths not exceeding 20 m.

Hollow-Stem Augers
A hollow stem auger looks a little like a large, non-tapered screw (see figure below). The auger flights are constructed around a hollow pipe. A drilling rig rotates the augers and a drilling bit on the end of the auger loosens the sediment which is then brought up to the surface by the rotating auger flights. The cuttings accumulate at the surface and must be shoveled away from the augers. The auger is advanced into the ground as it is rotated. A plug on the end of a rod inserted though the hollow stem may be screwed into the bit to seal the end of the opening and prevent sediment from entering inside the hollow stem. Hollow-stem auger drilling can be used to sample groundwater. At a selected horizon the plug at the end of the hollow stem is removed. A well point at the end of a rod is lowered to the bottom of the augers and is driven ahead of the bit by hammering or hydraulic pressure. A sample of groundwater is then taken following specified protocols. Soil and rock samples can be equally obtained by inserting a core sampler through the hollow-stem and retrieving a sample at a desired depth. The maximum depth of augered boreholes is for most practical purposes is about 50 m.

Mud and Air Rotary Drilling
A heavy drilling fluid is circulated in the borehole by pumping it down the inside of a hollow drill rod. A drill bit, attached at the end of the drill rod, rotates and cuts the formation rocks and advances the borehole downward. The drilling mud and rock cuttings, a type of mud, rises to the surface in the annular space between the borehole walls and the drill pipe and settles into a mud pond. The method of drilling just described is called standard rotary drilling. In reverse rotary drilling, the drilling fluid is injected through the annular space between the borehole walls and the drill pipe and exits, along with the rock cuttings, through the hollow drill rod. When drilling through bedrock, compressed air is preferred as the drilling fluid, sometimes assisted by a foaming agent that helps float cuttings through the surface. The injection of drilling fluids can have deleterious effects on ambient groundwater and adjacent formations. Careful selection of drilling fluids and ways to remove them prior to sample collection is called for in environmental drilling. In spite of the disturbance and possible contamination exerted during rotary drilling, in many hydrogeologic conditions, such as encountered in deep drilling through hard formations, this method is effective in constructing boreholes at a relatively rapid pace.
Cable-Tool Drilling
A heavy drill bit is placed at the end of an elongated hammer hanging from a cable. The drill rig repeatedly lifts and drops the hammer, which breaks up consolidated rock or loosens unconsolidated sediment. A steel casing is driven into the formation behind the drill bit. When the bottom of the casing fills with broken rock and sediment, the hammer and bit are removed, and a bottom bailer is used to remove the accumulated cuttings. Below the water table the groundwater and cuttings make a slurry. Above the water table, water must be added to make a slurry so that the bailer can be used. Because no slurry fluids need be circulated inside the borehole during drilling, induced formation contamination during drilling is minimized. Well points can be driven at the bottom of the borehole to collect groundwater samples. Likewise, a core sampler can be inserted upon removal of the drill tool to fetch rock samples.
Drive or Well Points
These are small-diameter wells, typically less than 1.5 inches in diameter, which are driven by hammering, hydraulic pressure, or vibration into soft soils to desired depths where groundwater samples need to be collected. A bailer is introduced through the center of a pipe to retrieve water samples. The pipe carves the borehole as it is driven down into the ground and serves as casing also. At the end of the pipe there is a sharp end, or point, which is screwed to a prepacked screen that allows the flow of water into the well while preventing well invasion by fine sediments.
Sonic/Rotosonic Drilling
Sonic drilling uses vibrations to move a drill casing deep into the subsurface. The technology does not use air or water to support the drilling process. Sonic drilling can be combined with casing rotation to enhance its penetration capability, giving rise to the rotosonic method. In either case, this drilling method is limited to soft sediments. When the matrix is suitable, the drilling process can yield undisturbed core samples. This drilling method can install neutron probes access casing, allowing continuous core and moisture profiles.
Slanted/Horizontal Drilling
This is typically a variation of rotary drilling in which the borehole and well casing are not vertical. Slanted drilling is a generic term encompassing well drilling at various angles from the vertical, including truly horizontal drilling. Slanted drilling uses the same method of ground penetration described earlier in rotary drilling. The innovative aspect in horizontal drilling technology is the use of a guidance system to direct the drilling bit along a pre-surveyed path. Casing installation, well screening and grouting, and screen packing follow specialized procedures. This type of well is particularly useful for installing recovery, remediation, and sparging systems under landfills or contaminated soils.
GROUNDWATER SAMPLING TECHNOLOGIES
Specialized sampling protocols must be followed with volatile and trace contaminants to ensure that they are preserved in proper phase and/or in solution prior to analytical testing. Micropurging is a type of low-volume sample collection that aims at exerting minimum disturbance in the sample. In micropurging, dedicated pumps are placed midway in screened intervals in a borehole. Pumping (or some other method of water collection) is done at a slow rate to prevent drawdown in the borehole and disturbance of the water column, thus yielding representative water samples at various depths. An alternative sampling method uses packers to isolate selected intervals inside a well that are then pumped individually to yield depth-screened samples. Special sampling devices are required to control the rate of sample collection. There are various nested-piezometer and well-sampling device configurations aimed at collecting water samples at single or multiple points in a layered aquifer. See Fetter (1994) for an elaboration of this subject matter.
Diffusion Samplers
Diffusion samplers are a relatively new sampling technology for collecting representative samples of volatile compounds in solution (Vroblesky et al., 1996). One or more samplers are lowered inside the borehole and can be located at selected depths. Therefore, the column of water, or waters originating from formations at different depths, can be sampled simultaneously. The sampler is made out of special semipermeable membranes that selectively allows for diffusion of targeted compounds (e.g., metals or VOCs) from the surrounding groundwater to its interior. The presence of chemicals inside the sampler can be used as a screening tool. The sampler's diffusion properties can be used to estimate the concentration of chemicals in groundwater by integrating the time-rate of chemical accumulation inside the sampler.
Dialysis Cells
The cells are separated vertically by flexible seals inside a borehole to provide multi-level information on groundwater quality. The sampler relies on movement of groundwater solutes into proprietary dialysis cells containing distilled water. The rate of solute accumulation inside the cells can be integrated over time to yield concentration in groundwater, or simply to screen for the presence of selected solutes in groundwater.
Open Bailer
This device is a rigid tube with an open top and closed bottom or a check valve at its bottom. It is attached to a line and lowered and raised by hand. It withdraws the sample from the top of the water column. A variant is the bailer with a foot valve, which fills through its bottom. The bottom-loading bailer has a spherical stopper, denser than water, which rests by gravity on a support built at the bottom of the bailer. As the bailer is lowered into the water column the sphere is displaced upwards, allowing water to enter the bailer. When the bailer is brought to a stop, the sphere falls by gravity onto its groove blocking the exit of captured water as the bailer is subsequently raised. The bottom-loading bailer can be attached to the lower end of a small-diameter tube and lowered to any desired depth inside the water column. By shaking the tube up and down in repetitive, continuous strokes, the tube fills from the bottom up until water flows at the surface where it can be stored in vials prior to analysis.
Kemmerer Bailer
This is a sampler originally developed for drawing water samples from lakes and oceans, that can be used equally as well as in wells. The sampler is lowered into the borehole in an open position until the desired depth is reached. At that point a weight is sent down on a cable suspending the bailer, triggering a spring-loaded device on the sampler to close it. The closed sampler is then raised to the surface by the cable and the water sample is withdrawn. The Kemmerer sampler can be used to collect water samplers at different depths so that geochemical logs can be developed.
Point-Source Bailer
This device has a check valve on both its top and bottom and can be lowered on a line to a given depth where the valves can be opened and closed by a cable. It can collect samples at any depth in the water column inside the borehole.
Gear Drive Pump
This device is similar to a traditional submersible electrical pump. There is a miniature electrical motor attached to the pump, which rotates a set of gears to drive the water sample up the discharge line via positive displacement. A continuous flow of water under positive pressure is developed.
Syringe Sampler
A syringe is attached to a length of tubing and lowered to a selected depth in the water column. Suction is applied to the tubing and the syringe, which is lowered empty. The syringe fills as the water comes into the needle because of the vacuum being developed by the suction on the tubing.
Bladder Pump
This sampler has a rigid tube containing an internal flexible bladder. There are check valves on either end of the rigid tube. When the bladder is deflated, water enters the lower end of the tube through the check valve. When the tube is full, the bladder is inflated with an inert gas pumped down from the surface. The bottom check valve closes, the top check valve opens, and the water sample flows up the discharge line. When the bladder deflates, the water in the discharge line cannot drain back into the rigid tube because of the check valve. The water is under positive pressure at all times and does not come into contact with the gas.
Helical Rotor Pump
This pump has a submersible electrical motor. It rotates a helical rotor stator, which drives water up the discharge line under positive pressure.
Gas-Driven Piston Pump
A piston that pumps the water is driven up and down by gas pressure from the surface. The gas does not contact the sample.
Peristaltic Pump
This is a pump located at the land surface. It is a self-priming vacuum pump that can draw a water sample up tubing under suction. Loss of volatile compound and dissolved gasses may occur due to the vacuum developed.
Submersible Centrifugal Pump
A submersible, electrical motor drives an impeller in the pump, which creates pressure and forces the water up a discharge line.
Gas Lift Pump
A constant stream of air is used to force the water up a discharge tube. The water comes into contact with the gas or air and oxidation and loss of volatile can occur.
Gas-Driven Pump
An inert gas is used to alternatively pressurize and depressurize a sampler chamber. The sample chamber has check valves to create a one-way flow of water up a discharge line. This is similar to a bladder pump except that there is no bladder, so the gas comes into contact with the water sample.
VADOSE ZONE CHARACTERIZATION TECHNOLOGIES
This section surveys key technologies for sampling gas, water, and chemicals in the vadose zone. For a review of water content and tension measuring devices such as time-domain reflectometry (for moisture content measurement) and tensiometers (for soil-water tension measurement), the reader is referred to the specialized literature for further details (see, e.g., Nielsen, 1991; Fetter, 1994; or Wilson et al., 1994).
Screw Sampler
The screw sampler is a tube of small diameter, typically less than 4 inches, driven mechanically downwards into the soil by hand or by a small portable gas engine. Its bottom end has an untapered screw-type shape, which penetrates into the ground as the sampler head at its top end is pushed down and rotated clockwise. Soil enters into the tube through its bottom, open end as the screw sampler is driven down. Soil cores can be retrieved from the screw sampler mechanically using smaller sampling corers.
Shelby Tube
This is a thin-walled tube that can be screwed to the end of a rod, lowered to the bottom of a borehole, and driven into cohesive sediments by applying downward pressure. The soil sample is extruded from the tube and trimmed into a permeameter or other apparatus for testing.
Split-Spoon Sampler
The split-spoon sampler consists of a split tube with walls thicker than a shelby tube. The two halves of the tube are matched and placed together by clamps located at their bottom and top ends. The assembled split-spoon sampler is screwed to a rod and lowered to the bottom of the borehole, where a 140 lb. Weight free falling through a distance of 30 inches is dropped repeatedly over the top of the rod to drive the sampler into the ground. The number of blows necessary to drive the sample every 6 inches into the soil is recorded until the sampler penetrates to a depth of 18 inches. At that point the sampler is removed from the formation and the sample removed by opening the two halves of the split-spoon sampler.
Cone Penetrometer Sampler
This type of sampler was introduced with the Geoprobe truck and related push-down technologies described earlier. A soil-sampling tube is screwed to the bottom end of a string of thick-walled, hollow rods, which are in turn screwed to each other to provide penetration depth. At its bottom end, the sampling tube has a solid drive point which penetrates the formation as pressure is applied at the top of the rods by a hydraulic and hammering system. Once the bottom of the sampling tube has reached the desired depth where the soil sample is to be collected, the drive point is freed to move upward to the top end of the sampling tube. The drive point is freed by removing a stopping pin located at the top of the sampling tube. The rod system is pushed down a distance equal to the length of the sampling tube, thus filling the tube with soil. The soil is packed inside a plastic sheath placed and securely fastened inside the sampling tube. Next the rods and sampling tube are pulled up by the Geoprobe's hydraulic lift until the sampling tube is brought to the surface. The plastic sheath with the soil sample is removed from within the sampling tube by applying a small force (e.g., gravity and gentle shaking). The soil sample is contained and tightly sealed inside the plastic sheath. The two exposed ends of the soil sample are capped and sealed after removal from the sampling tube. At this point the sample is ready for storage and/or analysis.
Soil-Gas Monitors
There are three main methods for gas sampling. In direct sampling, test boreholes are installed and soil samples are collected with a split-spoon sampler. The split-spoon sampler is opened and soil samples are collected with a small-diameter tube. These samples are placed in VOA (volatile organic analysis) vials. Volatile organics are then measured in the headspace in the vials. There is some gas loss in the sampling process introducing systematic underestimation errors in determining VOC concentrations. Soil cores can also be taken with Geoprobe or SCAPS platforms and kept tightly sealed prior to gas extraction and/or analysis in the laboratory.
Direct soil-gas probing relies on tubes that are pushed down into the soil (the push-down mechanism can be hydraulic, rotosonic, or mechanical). A suction source is applied inside the soil which draws gas into the tube and into a container or absorbed onto a porous matrix for later analysis. Alternatively, the captured gas can be fed directly into a field apparatus such as a gas chromatograph.
Passive gas chambers are excavated in the shallow vadose zone where a gas-collecting device or absorbent material is placed for days or weeks to capture gas mixtures emanating from the adjacent soil. The chamber or borings are sealed following the placement of the gas-collecting device. Some gas chambers have analytical devices that separate specific gases and provide a measure of their concentration in the soil-gas mixture.
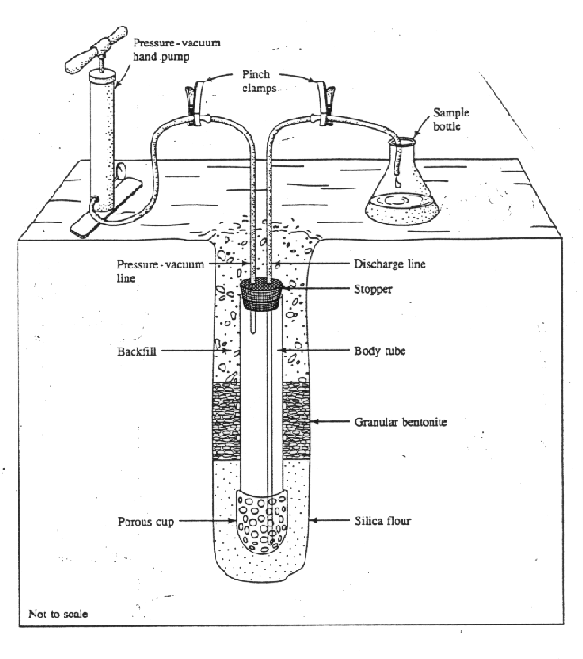
Lysimeter
The suction lysimeter consists of a hollow tube with a porous cup at the end. The hollow tube has a stopper at its top end which extends to the surface (see figure below). The tube is made of PVC or stainless steel. The porous cup can be ceramic, nylon, PFTE, or fritted stainless steel. The porous cup is inserted in the soil at a desired depth. The boring around the ceramic cup is typically filled up with a fined-textured silica matrix or fine-textured soil that establish a good hydraulic contact between the cup and the adjacent soil from which water is extracted. Suction is applied to the hollow tube with a vacuum pump attached to a small-diameter tubing that protrudes into the top part of the lysimeter through the stopper. Once a desired suction is reached, the vacuum line is closed with a pinch clamp. As long as the suction inside the lysimeter is greater than the soil-moisture tension in the soil, soil water, if available, is drawn into the lysimeter. Once hydraulic equilibrium is reached, the soil-water flux into the lysimeter ceases. A second, long tube that reaches the bottom of the lysimeter, and which is kept closed with a pinch clamp up to this point, is opened to discharge the lysimeter water into a sample bottle. The pinch clamp in the vacuum line is removed and a pressure pump replaces the vacuum pump. Pressure is then applied to the lysimeter and the water in the lysimeter is drained to the sample bottle via the long drainage tube.
B. ANALYTICAL TECHNOLOGIES
Atomic-Absorption Spectrometry (AA)
Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids, the analyte atoms or ions must be vaporized in a flame or graphite furnace. The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. The analyte concentration is determined from the amount of absorption. Applying the Beer-Lambert law directly in AA spectroscopy is difficult due to variations in the atomization efficiency from the sample matrix, and non-uniformity of concentration and path length of analyte atoms (in graphite furnace AA). Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration.

Atomic Emission Spectrometry (AES, OES)
Atomic emission spectroscopy (AES or OES) uses quantitative measurement of the optical emission from excited atoms to determine analyte concentration. Analyte atoms in solution are aspirated into the excitation region where they are desolvated, vaporized, and atomized by a flame, discharge, or plasma. These high-temperature atomization sources provide sufficient energy to promote the atoms into high energy levels. The atoms decay back to lower levels by emitting light. Since the transitions are between distinct atomic energy levels, the emission lines in the spectra are narrow. The spectra of multi-elemental samples can be very congested, and spectral separation of nearby atomic transitions requires a high-resolution spectrometer. Since all atoms in a sample are excited simultaneously, they can be detected simultaneously, and is the major advantage of AES compared to atomic-absorption (AA) spectroscopy.

Atomic-Fluorescence Spectroscopy (AFS)
Atomic fluorescence is the optical emission from gas-phase atoms that have been excited to higher energy levels by absorption of electromagnetic radiation. The main advantage of fluorescence detection compared to absorption measurements is the greater sensitivity achievable because the fluorescence signal has a very low background. The resonant excitation provides selective excitation of the analyte to avoid interference. AFS is useful to study the electronic structure of atoms and to make quantitative measurements. Analytical applications include flames and plasmas diagnostics, and enhanced sensitivity in atomic analysis. Because of the differences in the nature of the energy-level structure between atoms and molecules, discussion of laser-induced fluorescence (LIF) from molecules is outside the scope of this review. The reader is referred to the specialized literature for a more in-depth survey of these topics.
Instrumentation
Analysis of solutions or solids requires that the analyte atoms be desolvated, vaporized, and atomized at a relatively low temperature in a heat pipe, flame, or graphite furnace. A hollow-cathode lamp or laser provides the resonant excitation to promote the atoms to higher energy levels. The atomic fluorescence is dispersed and detected by monochromators and photomultiplier tubes, similar to atomic-emission spectroscopy instrumentation.
Electrical Conductivity Sensor
This device can be mounted in a cone penetrometer, a down-hole probing device, or used in a hand-held fashion. The sensor or probe consists of two electrodes, one positive and the other negative, which constitute the terminals of a battery of specified voltage in a very simple electrical circuit. When placed in a soil or groundwater solution, an electrical current is generated between the electrodes due to the migration of anions and cations present in solution towards the respective electrode. The magnitude of the electrical current between the electrodes measures the presence of ionic solutes in the water solution, and thus, is an expeditious indicator of water quality. The conductivity of a water solution is a measure of its ability to transmit an electrical current. Thus, high "conductivity" of the water solution implies high solute concentrations, and vice versa. Strictly speaking, conductivity is defined as the inverse of resistivity, where the latter is measured in ohms× cm. The resistivity r is related to the resistance R by R = r A/L, where A is the cross-sectional area through which electrical current passes and L is the distance between electrodes. It is known that the voltage applied by the battery, V, is related to the electrical current that circulates between electrodes, I, and the resistance, R, by the relationship V = I*R. The electrical conductance is given by R-1, and is expressed in mhos (1 mho = 1 ohm-1). Thus, if the applied voltage is V and the measured current is I, the electrical conductance, C, is C = I/V, in ohm-1, while the electrical conductivity would be c = C L/A, in mhos/cm (1 mho/cm = 1 ohm-1/cm).
Explosive Sensor
This is a safety device. It is used above-ground or in-trench. It measures the concentrations of elements, such as gases, thus enabling the prediction of explosion potential from buried compounds (e.g., ammunition).
Fiber Optic Sensor
This device is attached to a cone penetrometer. It uses the differential capacity of contaminants to absorb light in different ranges of the electromagnetic spectrum to identify chemicals in the subsurface. Several contaminants can be identified simultaneously by the use of a beam containing a broad spectrum of wavelengths.
Free Product Sensor
This device is lowered in a borehole or trench. It measures the thickness of the contaminant ("free product", typically light petroleum hydrocarbons) which accumulates over the groundwater, which is more dense. It is also known as an interface meter.
Gas Chromatography (GC)
Introduction
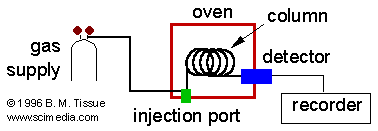
Gas chromatography is a chromatographic technique that can be used to separate volatile organic compounds. A gas chromatograph consists of a flowing mobile phase, an injection port, a separation column containing the stationary phase, and a detector. The organic compounds are separated due to differences in their partitioning behavior between the mobile gas phase and the stationary phase in the column.
Instrumentation
Mobile phases are generally inert gases such as helium, argon, or nitrogen. The injection port consists of a rubber septum through which a syringe needle is inserted to inject the sample. The injection port is maintained at a higher temperature than the boiling point of the least volatile component in the sample mixture. Since the partitioning behavior is dependent on temperature, the separation column is usually contained in a thermostat-controlled oven. Separating components with a wide range of boiling points is accomplished by starting at a low oven temperature and increasing the temperature over time to elute the high-boiling point components. Most columns contain a liquid stationary phase on a solid support. Separation of low-molecular weight gases is accomplished with solid adsorbents. Specific GC Columns and GC detectors are described in the specialized literature.
High-Performance Liquid Chromatography (HPLC)
Introduction
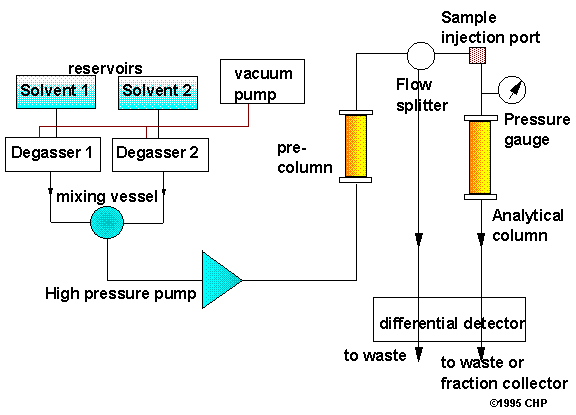
High-performance liquid chromatography (HPLC) is a form of liquid chromatography to separate compounds that are dissolved in solution. HPLC instruments consist of a reservoir of mobile phase, a pump, an injector, a separation column, and a detector. Compounds are separated by injecting a plug of the sample mixture onto the column. The different components in the mixture pass through the column at different rates due to differences in their partitioning behavior between the mobile liquid phase and the stationary phase.
Instrumentation
Solvents must be degassed to eliminate formation of bubbles. The pumps provide a steady high pressure with no pulsating, and can be programmed to vary the composition of the solvent during the course of the separation Detectors rely on a change in refractive index, UV-VIS absorption, or fluorescence after excitation with a suitable wavelength. There are different types of HPLC columns, and the reader is referred to the specialized literature for further details.
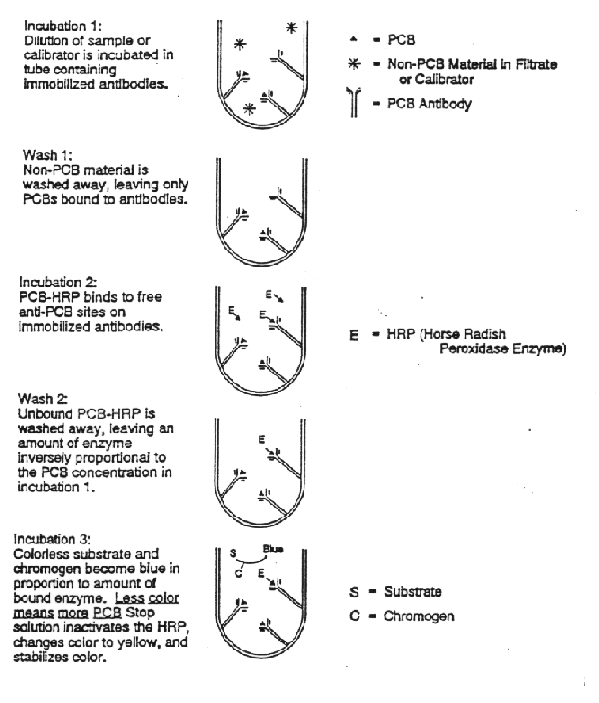
Immunoassay Test Kit
Immunoassay test kits for PCBs have proven somewhat successful for in-situ screening of total PCB contaminants. An example is the EnvirogaurdTM PCB test kit shown in the figure below (US EPA, 1994). Soil extracts are added to test tubes coated with antibodies that bind PCB molecules. The soil extracts are washed away after incubation, and the PCB conjugate, a horse raddish peroxidase enzyme, mimics free PCB molecules and is added to the tubes. Unoccupied antibody binding sites bind the PCB conjugate. Excess PCB conjugate is washed away. An enzime substitute and a coloring agent are then added to the test tube. The color intensity of the test tube is measured at 405 nanometers using a small, portable spectrophotometer. The color intensity in inversely proportional to PCB concentration in the soil sample. The results obtained from the soil samples are compared against three calibrators of 5, 10, and 50 ppm. This type of test is called a competitive enzyme-linked immunosorbent assay (ELISA).
PCB concentrations are semiquantitatively classified as below 5 ppm, between 5 and 10 ppm, and greater than 50 ppm. Up to six sample analyses (in duplicate) can be performed in about 15 to 20 minutes.
Infrared Absorption Spectroscopy (IR)
IR spectroscopy is the measurement of the wavelength and intensity of the absorption of mid-infrared light by a sample. Mid-infrared light (2.5 - 50 µm, 4000 - 200 cm-1) is energetic enough to excite molecular vibrations to higher energy levels. The wavelength of IR absorption bands are characteristic of specific types of chemical bonds, and IR spectroscopy finds its greatest utility for identification of organic and organometallic molecules.
Ion-Trap Mass Spectrometry
The ion-trap mass spectrometer uses three electrodes to trap ions in a small volume. The mass analyzer consists of a ring electrode separating two hemispherical electrodes. A mass spectrum is obtained by changing the electrode voltages to eject the ions from the trap. The advantages of the ion-trap mass spectrometer include compact size, and the ability to trap and accumulate ions to increase the signal-to-noise ratio of a measurement.
Laser Spectroscopy
Lasers revolutionized spectroscopy when they were invented in the early 1960's. They provide an electromagnetic radiation source that is intense, directional, and coherent. Lasers allow high-resolution absorption and emission studies. More information on these processes can be found in atomic fluorescence (AFS) and molecular laser-induced fluorescence (LIF) documents. The discussions below describe some of the new types of spectroscopies that were not possible using conventional light sources.
Doppler-free laser spectroscopy
Doppler broadening arises from the distribution of absorption (or emission) frequencies of atoms (or molecules). The distribution of frequencies occurs because the atoms have a distribution of velocities relative to the laser beam and are therefore Doppler shifted by: w = wo ± k * v, where w is the laser frequency, wo is the transition frequency, k is the wavevector of the radiation, and v is the velocity of the atom. Removing Doppler broadening allows measurement of the natural linewidth, and shows any underlying fine structure such as isotope shifts, hyperfine splitting, and Zeeman splitting.
Saturation spectroscopy
Counter-propagating laser beams produce a Doppler-free saturation dip (Lamb dip) in the center of a Doppler-broadened line. When the laser frequency is tuned off line center, one beam interacts with +k*v atoms, while the beam propagating in the opposite direction interacts with -k*v atoms. At the center of the Doppler line both laser beams interact with the same velocity group (k*v=0, i.e., atoms moving perpendicular to the laser beams). When the laser intensity is high enough to saturate the transition, the counter-propagating beam adds no additional excitation of the k*v=0 atoms and the overall observed signal is less than that for the case when exciting k*v atoms. This decrease in the signal appears as a narrow Lamb dip.
Two-photon absorption
For atoms or molecules with convenient two-photon transitions, the Doppler width can be eliminated by using counter-propagating beams. In the atomic frame of reference, the two laser beams appear at frequencies of wo(1- k*v/c) and wo(1+ k*v/c), where wo is the frequency halfway to the two-photon level. The velocity dependence cancels out and the net result is that all atoms will absorb photons with a total energy of the two-photon transition. Due to absorption of two photons travelling in the same direction, this method will have some residual absorption of the full Doppler width.
Mass Spectrometry (MS)
Mass spectrometers use the difference in mass-to-charge ratio (m/e) of ionized atoms or molecules to separate them from each other. Mass spectrometry is therefore useful for quantification of atoms or molecules and also for determining chemical and structural information about molecules. Molecules have distinctive fragmentation patterns that provide structural information to identify structural components.
The general operation of a mass spectrometer is:
1. Create gas-phase ions
2. Separate the ions in space or time based on their mass-to-charge
ratio
3. Measure the quantity of ions of each mass-to-charge ratio
The ion separation power of a mass spectrometer is described by its resolution, which is defined as R = m / D m, where m is the ion mass and D m is the difference in mass between two resolvable peaks in a mass spectrum. For example, a mass spectrometer with a resolution of 1000 can resolve an ion with a m/e of 100.0 from an ion with a m/e of 100.1.
Instrumentation
In general a mass spectrometer consists of an ion source, a mass-selective analyzer, and an ion detector. The magnetic-sector, quadrupole, and time-of-flight designs also require extraction and acceleration ion optics to transfer ions from the source region into the mass analyzer. The details of mass analyzer designs, descriptions of sample introduction/ionization and ion detection can be found in the specialized literature.
Membrane-based devices
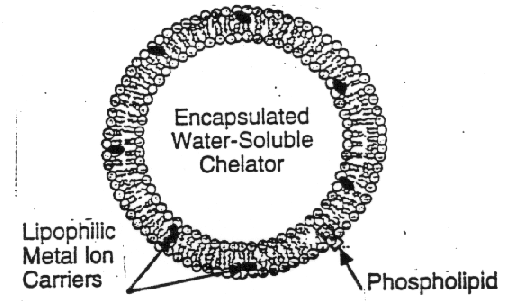
Membrane testing devices utilize preferentially permeable membranes, which permit the free passage by diffusion of some materials and restricts the passage of others. In our case, the materials that diffuse through such membranes are contaminants present in a water solution. The amount of material that passes through the membrane is related to its concentration in solution, and this forms the basis for determining in-situ concentrations based on the application of membrane technology. Preferentially permeable membranes have been developed for a number of contaminants, such as TCE, PCB, and metals. A recent development in membrane-based testing technology is the so-called vesicle luminescence based detection system for toxic metal ions (Walsh and Monbouquette, 1993). Vesicles are spherical capsules on the order of 100 nm in diameter. The vesicle walls are cell membranes composed primarily of phospholids. The phospholids are non-covalently assembled two-tailed surfactants, i.e., molecules with a polar, phosphorous containing, water soluble headgroup and two insoluble hydrocarbon tails. In an aqueous environment, phospholids assemble into lipid bilayer structures where the polar head groups are oriented toward the surrounding aqueous medium and the hydrocarbon tails interact with themselves. A vesicle (also called spheroidal capsule or liposome) is shown in the figure below. The hydrocarbon core of the 4 nm-thick lipid bilayer membrane is virtually impermeable to charged or large polar species, thus transmembrane transport is governed by selective carriers, such as ionophores for metal ions, that shield solute charge or polarity and permit transport across the membrane. A strong chelating agent such as nitrilotriacetate (NTA) encapsulated within the vesicle maintains the internal free-metal-ion concentration at vanishingly low levels thereby providing the driving force for metal-ion uptake. A fluorescent chelator inside the vesicle will be paired with the metal-ion carrying ionophores a nd t he excitation and emission spectra of the chelator is calibrated to detect ionic metals at the picomolar level (single digit ppb or lower concentrations. Metals being tested for with the developing vesicular membrane technology include Cd+2, Pb+2, Cu+2, and Zn+2.
Near-Infrared Absorption Spectroscopy (NIR)
NIR spectroscopy is the measurement of the wavelength and intensity of the absorption of near-infrared light by a sample. Near-infrared light spans the 800 nm - 2.5 µm (12,500 - 4000 cm-1) range and is energetic enough to excite overtones and combinations of molecular vibrations to higher energy levels. NIR spectroscopy is typically used for quantitative measurement of organic functional groups, especially O-H, N-H, and C=O. Detection limits are typically 0.1% and applications include pharmaceutical, agricultural, polymer, and clinical analysis.
Instrumentation
The components and design of NIR instrumentation are similar to uv-vis absorption spectrometers. The light source is usually a tungsten lamp and the detector is usually a PbS solid-state detector. Sample holders can be glass or quartz and typical solvents are CCl4 and CS2. The convenient instrumentation of NIR spectroscopy compared to IR spectroscopy makes it much more suitable for on-line monitoring and process control.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclei with an odd number of protons, neutrons, or both, have an intrinsic nuclear spin.
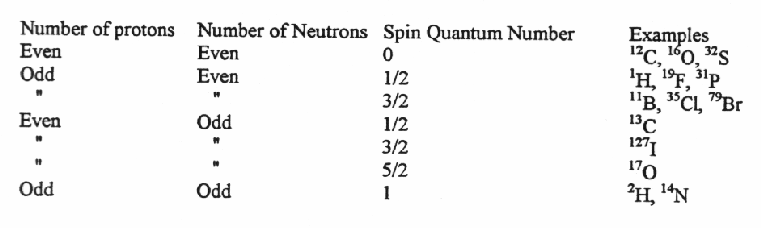
When a nucleus with a non-zero spin is placed in a magnetic field, the nuclear spin can align in either the same direction or in the opposite direction as the field. These two nuclear spin alignments have different energies and application of a magnetic field lifts the degeneracy of the nuclear spins. A nucleus that has its spin aligned with the field will have a lower energy than when it has its spin aligned in the opposite direction to the field.
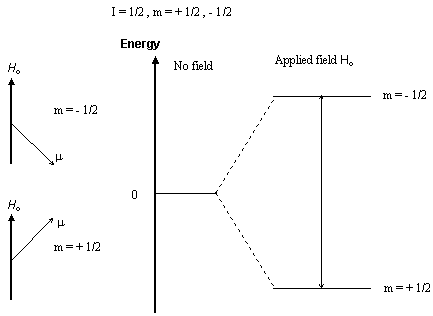
Nuclear magnetic resonance (NMR) spectroscopy is the absorption of radiofrequency radiation by a nucleus in a strong magnetic field. Absorption of the radiation causes the nuclear spin to realign or flip in the higher-energy direction. After absorbing energy the nuclei will reemit RF radiation and return to the lower-energy state.
The energy of a NMR transition depends on the magnetic-field strength and a proportionality factor for each nucleus called the magnetogyric ratio. The local environment around a given nucleus in a molecule will slightly perturb the local magnetic field exerted on that nucleus and affect its exact transition energy. This dependence of the transition energy on the position of a particular atom in a molecule makes NMR spectroscopy extremely useful for determining the structure of molecules.
Instrumentation
There are two NMR spectrometer designs, continuous-wave (CW), and pulsed or Fourier-transform (FT-NMR). CW-NMR spectrometers have largely been replaced with pulsed FT-NMR instruments. However due to the lower maintenance and operating cost of CW instruments, they are still commonly used for routine 1H NMR spectroscopy at 60 MHz. (Low-resolution CW instruments require only water-cooled electromagnets instead of the liquid-He-cooled superconducting magnets found in higher-field FT-NMR spectrometers.) These two spectrometer designs are described in separate CW-NMR and FT-NMR documents.
Optical Radiation Detectors
Introduction
Detectors convert light energy to an electrical signal. In spectroscopy, they are typically placed after a wavelength separator to detect a selected wavelength of light. Different types of detectors are sensitive in different parts of the electromagnetic spectrum.
Index of radiation detectors
Charge-coupled detector array (CCD)
Semiconductors (photodiodes and photovoltaics)
Photodiode array (PDA)
Photomultiplier tube (PMT)
Photo-ionization Detector
Introduction
The reason to use more than one kind of detector for gas chromatography is to achieve selective and/or highly sensitive detection of specific compounds encountered in particular chromatographic analyses. The selective determination of aromatic hydrocarbons or organo-heteroatom species is the job of the photoionization detector (PID). This device uses ultraviolet light as a means of ionizing an analyte exiting from a GC column. The ions produced by this process are collected by electrodes. The current generated is therefore a measure of the analyte concentration.
Selective detection using a PID
Here is an example of selective PID detection: Benzene's boiling point is 80.1 degrees C and its IP is 9.24 ev. (Check the CRC Handbook 56th ed. page E-74 for IPs of common molecules.) This compound would respond in a PID with an UV lamp of 9.5 ev (commercially available) because this energy is higher than benzene's IP (9.24). Isopropyl alcohol has a similar boiling point (82.5 degrees C) and these two compounds might elute relatively close together in normal temperature programmed gas chromatography, especially if a fast temperature ramp were used. However, since isopropyl alcohol's IP is 10.15 ev this compound would be invisible or show very poor response in that PID, and therefore the detector would respond to one compound but not the other.
Instrumentation
Since only a small (but basically unknown) fraction of the analyte molecules are actually ionized in the PID chamber, this is considered to be a nondestructive GC detector. Therefore, the exhaust port of the PID can be connected to another detector in series with the PID. In this way data from two different detectors can be taken simultaneously, and selective detection of PID responsive compounds augmented by response from, say, an FID or ECD. The major challenge here is to make the design of the ionization chamber and the downstream connections to the second detector as low volume as possible (read small diameter) so that peaks that have been separated by the GC column do not broaden out before detection.
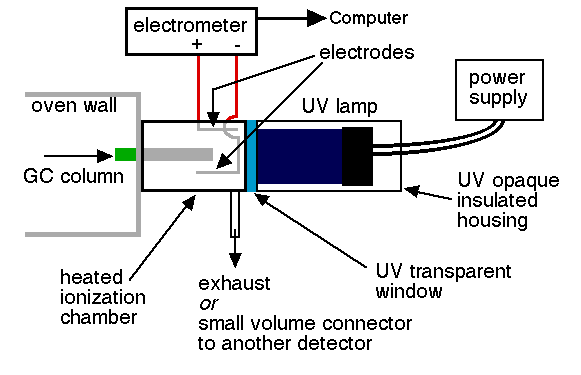
Piezoelectric sensor
This is a sensor that detects the air-water interface in a borehole. It is used to determine the depth to water, or the absolute elevation of the water level, in a borehole. It is based on the basic principle that there is no electrical current between the terminals attached to a battery when the circuit is not closed due to the physical separation between the electrodes by air, which a very poor conductor. When the electrodes enter water (a good conductor), the circuit is closed and current flows, activating a galvanometer or beeper, which then signals the presence of the air/water interface. The position of the water level is determined by physical measurement with a tape or similar device.
Radiation detectors
Introduction
Radiation is produced by the disintegration or decay of unstable atomic nuclei. The rays emitted in radioactivity are classified as a rays, b rays, and g rays. a rays or particles are positively charged and are simply the nuclei of helium atoms. That is, an a ray consists of two protons and two neutrons bound together. b rays are negatively charged. They are electrons, identical to those that orbit the nucleus (but they are created within the nucleus itself). g rays are neutral in charge. They are high-energy protons. A few counters of radioactive particles are reviewed below (synthesized from Giancoli, 1991).
Geiger counter
It consists of a cylindrical metal tube filled with gas. A long wire runs down the center of the tube and is kept at a high positive voltage (approximately 1000 V) with respect to the outer cylinder. The voltage is just slightly less than that required to ionize the gas atoms. When a charged particle (produced by radioactivity) enters through a thin window at one end of the tube, it ionizes a few atoms of the gas. The freed electrons are attracted toward the positive wire and as they are accelerated they strike and ionize additional atoms. A surge of electrons is produced, and when they reach the wire anode they produce a voltage pulse. The pulse is amplified and sent to an electronic counter, which counts how many particles have been detected. Alternatively, the pulses can be sent to a loudspeaker and each individual detection of a radioactive particle is heard as a click.

Scintillation counter
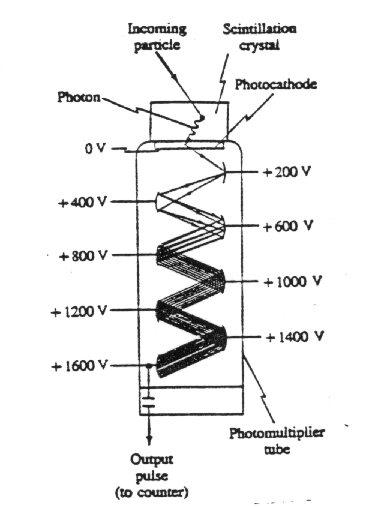
It makes use of a solid, liquid, or gas known as a scintillator or phosphor. The atoms of a scintillator, being easily excited when struck by an incoming radioactive particle, emit visible light when they return to other ground states. Typical scintillators are crystals of NaI and certain plastics. One face of a scintillator is cemented to a photomultiplier tube, and the whole is wrapped with opaque material to keep it light-tight or is placed within a light-tight container. The photomultiplier (PM) tube converts the energy of the scintillator-emitted photons(s) into an electrical signal. A PM is a vacuum tube containing several electrodes (typically 8 to 14), called dynodes, which are maintained at successively higher voltages as shown in the figure below. At its top surface is a photoelectric surface called the photocathode, whose function is low enough that electrons are easily released when struck by a photon from the scintillator. Such an electron is accelerated toward the first dynode. When it strikes the first dynode, the electron has acquired sufficient kinetic energy so that it can eject two to five more electrons. These, in turn, are accelerated to the second dynode, and a multiplication process begins. The number of electrons striking the last dynode may be 106 or more. Thus the passage of a particle through the scintillator results in an electric signal at the output of the PM tube that can be sent to an electronic counter just as for a Geiger tube. Because a scintillator crystal is much more dense than the gas of a Geiger counter, it is a more efficient detector. This is especially true for g rays, which interact less with matter than b rays. In tracer work and biological experiments, liquid scintillators are often used. Radioactive samples are placed directly in small bottles containing the liquid scintillator. This is particularly convenient for detection of b rays from tritium and carbo n-14, which have very low energies and have difficulty passing through the outer covering of a crystal scintillator or Geiger tube. A PM tube is still used to produce the electric signal.
Semiconductor detector
A semiconductor detector consists of a reverse-biased pn junction diode. A particle passing through the junction can excite electrons into the conduction band, leaving holes in the valence band. The freed charges produce a short electrical pulse that can be counted just as for Geiger and scintillation counters.
Raman Spectroscopy
Raman spectroscopy is the measurement of the wavelength and intensity of inelastically scattered light from molecules. The Raman scattered light occurs at wavelengths that are shifted from the incident light by the energies of molecular vibrations. The mechanism of Raman scattering is different from that of infrared absorption, and Raman and IR spectra provide complementary information. Typical applications are in structure determination, multicomponent qualitative analysis, and quantitative analysis.
Instrumentation
The most common light source in Raman spectroscopy is an Ar-ion laser. Resonance Raman spectroscopy requires tunable radiation and sources are Ar-ion-laser-pumped CW dye lasers, or high-repetition-rate excimer-laser-pumped pulsed dye lasers. Because Raman scattering is a weak process, a key requirement to obtain Raman spectra is that the spectrometer provide a high rejection of scattered laser light. New methods such as very narrow rejection filters and Fourier-transform techniques are becoming more widespread.
Sensors (pH, electrical conductivity, salinity, dissolved oxygen, etc.)
A sensor is a device that produces a measurable response to a change in a physical condition, such as temperature or thermal conductivity, or to a change in chemical concentration. Sensors are particularly useful for making in-situ measurements such as in industrial process control, medical applications, or environmental monitoring. A sensor is usually packaged as a complete unit. Widely used sensors in groundwater measure temperature, electrical conductivity, salinity, pH, dissolved oxygen, and other parameters.
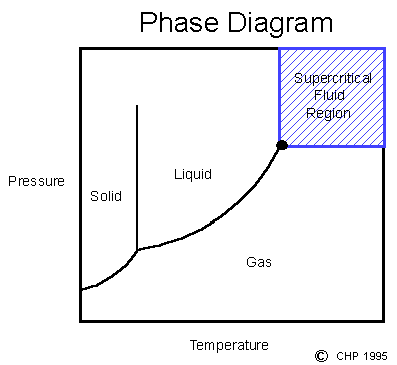
Supercritical Fluids
Supercritical fluids are produced by heating a gas above its critical temperature or compressing a liquid above its critical pressure (see phase diagram below). Under these conditions, the molar volume is the same whether the original form was a liquid or a gas.
Supercritical Fluid Extraction
Supercritical fluids can be used to extract analytes from samples. The main advantages of using supercritical fluids for extractions are that they are inexpensive, contaminant free, and produce no environmental hazard for disposal. For these reasons supercritical fluid CO2 is the reagent used to extract caffeine from coffee.
The properties of supercritical fluids also provide some advantages for analytical extractions. Supercritical fluids can have solvating powers similar to organic solvents, but with higher diffusivities, lower viscosity, and lower surface tension. The solvating power can be adjusted by changing the pressure or temperature, or adding modifiers to the supercritical fluid. A common modifier is methanol (typically 1-10%) which increases the polarity of supercritical CO2.
Thin-Layer Chromatography (TLC)
Thin-layer chromatography (TLC) is a chromatographic technique that is useful for separating organic compounds. Because of the simplicity and rapidity of TLC, it is often used to monitor the progress of organic reactions and to check the purity of products.
Method
Thin-layer chromatography consists of a stationary phase immobilized on a glass or plastic plate and a solvent. The sample, either liquid or dissolved in a volatile solvent, is deposited as a spot on the stationary phase. The constituents of a sample can be identified by simultaneously running standards with the unknown. One edge of the plate is then placed in a solvent reservoir and the solvent moves up the plate by capillary action. When the solvent front reaches the other edge of the stationary phase, the plate is removed from the solvent reservoir. The separated spots are visualized with ultraviolet light or by placing the plate in iodine vapor. The different components in the mixture move up the plate at different rates due to differences in their partitioning behavior between the mobile liquid phase and the stationary phase.
Titration
Titration is the quantitative measurement of an analyte in solution by completely reacting it with a reagent. The point at which all of the analyte is consumed is called the endpoint and is determined by some type of indicator that is also present in the solution. For acid-base titrations, indicators are available that change color when the pH changes. When all of the analyte is neutralized, further addition of the titrant causes the pH of the solution to change causing the color of the indicator to change. The analyte concentration is calculated from the reaction stoichiometry and the amount of reagent that was required to react with all of the analyte.
Instrumentation
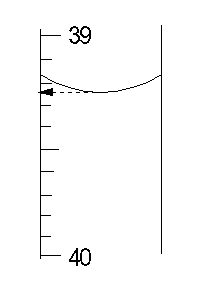
Manual titration is done with a buret, which is a long graduated tube to hold the titrant. The amount of titrant used in the titration is found by reading the volume of titrant in the buret before beginning the titration and when the endpoint is reached, and taking the difference. The most important factor for making accurate titrations is to read the buret volumes reproducibly. The figure shows how to do so by using the bottom of the meniscus to read the reagent volume in the buret.
For repetitive titrations, autotitrators with microprocessors are available that deliver the titrant, stop at the endpoint, and calculate the concentration of the analyte. The endpoint is usually detected by some type of electrochemical measurement. Some examples of titrations for which autotitrators are available include:
Acid or base determination by pH measurement with potentiometric detection.
Determination of water by Karl Fischer reagent (I2 and SO2
in methyl alcohol and pyridine) with colorimetric detection.
Determination of Cl in aqueous solution with phenylarsene oxide using
amperometric detection.
Ultraviolet and Visible Absorption Spectroscopy (uv-vis)
UV-vis spectroscopy is the measurement of the wavelength and intensity of absorption of near-ultraviolet and visible light by a sample. Ultraviolet and visible light are energetic enough to promote outer electrons to higher energy levels. UV-vis spectroscopy is usually applied to molecules and inorganic ions or complexes in solution. The uv-vis spectra have broad features that are of limited use for sample identification but are very useful for quantitative measurements. The concentration of an analyte in solution can be determined by measuring the absorbance at some wavelength and applying the Beer-Lambert law.

Instrumentation
The light source is usually a hydrogen or deuterium lamp for uv measurements and a tungsten lamp for visible measurements. The wavelengths of these continuous light sources are selected with a wavelength separator such as a prism or grating monochromator. Spectra are obtained by scanning the wavelength separator and quantitative measurements can be made from a spectrum or at a single wavelength.
X-ray Fluorescence
X-ray fluorescence is a spectroscopic method that is commonly used for solids in which secondary x-ray emission is generated by excitation of a sample with x-rays. The x-rays eject inner-shell electrons. Outer-shell electrons take their place and emit photons in the process. The wavelength of the photons depends on the energy difference between the outer-shell and inner-shell electron orbitals. The amount of x-ray fluorescence is very sample dependent and qualitative analysis requires calibration with standards that are similar to the sample matrix.
Instrumentation
Solid samples are usually powdered and pressed into a wafer or fused in a borate glass.
NUMERICAL/SPATIAL/STATISTICAL TECHNOLOGIES
Geostatistical/statistical
Geostatistics is a branch of statistics that studies the spatial and temporal distribution of earth processes, such as the spreading of pollutants in the subsurface (see Loaiciga et al., 1989a,b). Statistical analysis is broader in scope, and it includes the methods germane to geostatistics (such as kriging and co-kriging) as well as all other established methods of statistical inference, probability theory, and time series analysis to study the point, spatial, and spatial/temporal distribution of earth processes. In the context of subsurface contamination and environmental monitoring studies, geostatistical/statistical methods are routinely applied to: interpolate values at points where measurements were not made, test for the presence of trends in contaminant concentrations, conduct statistical inference on the properties of chemical concentrations in soil and groundwater, and estimate risk of contaminant migration to sensitive areas. The methods of geostatistics/statistics have been coded in public-domain and commercial software.
Flow and Transport models
There are analytical and numerical models that predict soil water flow, groundwater flow, solute, heat, and multiphase/multi-species transport in the subsurface. These models can be classified according to the method of solution (e.g., numerical, analytical, experimental), the target variable being predicted (e.g., water, gas, heat, biological species, chemicals), the nature of the transport process (e.g., single phase, multi-phase, single-species, multi-species, iso-thermal, temperature-dependent, compressible-matrix, incompressible-matrix, constant-density, variable-density, etc.), the degree of saturation and texture of the porous matrix (e.g., vadose zone, saturated zone, primary-porosity matrix, fractured bedrock), the space dimensionality (one-, two-, and three-dimensional fields), or the temporal variability of the studied process (steady-state, unsteady-state). The list of models available is too large to be included here. Two public-domain models that are widely used are the three-dimensional/finite difference groundwater flow model, MODFLOW (McDonald and Harbaugh, 1988) and the solute transport/variable density model HS3D, both developed by the U.S. Geological Survey. Many of these special-purpose models are being integrated into large software packages that combine their individual capabilities. The most versatile subsurface contamination models currently in use are numerical finite-difference models that can simulate fairly realistic field conditions in relatively inexpensive personal computers. Flow and transport models are particularly useful in risk assessment studies of subsurface contamination, as they can simulate the spatial and temporal spreading of chemical constituents under a wide spectrum of natural, remediation, or clean-up conditions.
Geographic Information Systems/Expert-Spatial Decision Support systems
A Geographic Information System (GIS) is a complex piece of software, capable of carrying out spatial data storage, manipulation, and display. GIS's are gaining prominence as a powerful tool for a broad range of environmental management tasks (Burroughs, 1986; Goodchild et al., 1993; Leipnik, 1995; Loaiciga 1995). GIS's can establish and maintain explicit links between geographically referenced entities (e.g., groundwater wells, soils sampling locations) and their attributes (e.g., well depths, soil contaminant concentrations). In addition, they have projection management and cartographic transformation capabilities, and store spatial topology. These capabilities enable such useful functions such as the overlay of thematic maps (e.g., soils, geology, vegetation, hydrogaphy, and land use maps), buffer zone generation, proximity intersection/inclusion analysis of spatial features, least-cost path analysis, and a wide array of topographic and terrain analysis capabilities. GIS's have been interfaced with ground flow and transport models in large-scale simulations to facilitate and enhance data input and output management, quality control, and display.
A Spatial Decision Support System is a computer-based system designed to provide the following capabilities: (i) an interactive graphical user interface (GUI) for data input and output, (ii) data storage, (iii) spatial and numerical analysis, and (iv) graphical visualization and cartographic products. The high-end SDSS's are those that, in addition to the previous four capabilities, include an expert system for the purpose of quality assurance and quality control and/or selection of remediation strategies based on available data and pre-programmed expert knowledge into the expert system.