| An Inexpensive X Ray Machine
The following is taken in full from
Section IX. Optics, Heat, and Electronics;
Chapter 3. An Inexpensive X-ray Machine
The Scientific American Book of Projects for The Amateur
Scientist
Library of Congress Card Catalog Number: 60-14286
© Copyright 1960 by C. L. Strong
3
An Inexpensive X-ray Machine
From an old radio tube, some copper wire, and other
inexpensive materials — total cost: roughly $20 — you can
construct an X-ray machine that will make good pictures through an
inch of wood. SAFETY MEASURES THAT YOU MUST OBSERVE. Notes on Röntgen's
invention. Highlights of X-ray theory.
Harry Simons of 118 Windsor Street, Kearny, N.J., is a lonely
amateur scientist. “For 23 years,” he writes, “I have been
dabbling in the X-ray portion of the electromagnetic spectrum
without once coming across a fellow amateur. Thousands of
enthusiasts can be found in the region of radio waves, of light
and of gamma rays. But none of them come to play in my back yard.
If the prospect of exploring fresh electromagnetic territory
sounds interesting to any of these amateurs, I can promise good
hunting in the
-centimeter region — and for a total investment of less than
$20.”
As a lure Simons offers the collection of radiographs
reproduced in Figures 225, 226, 227 and 228. He takes special
pride in the one which shows screws embedded in an inch-thick
block of wood. This shot resulted from his first experiment with
X-rays and illustrates what can happen when a fellow with a sharp
eye follows a happy hunch.
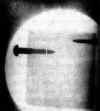
225 Simon's radiograph of two screws imbedded in an inch-thick
block of wood

226 Radiograph of the plug from an electric flatiron

227 bones of fish revealed by homemade X-ray machine

228 Effectiveness of Simon's X-ray machine in penetrating the
steel leaves of a thickness ranging from .002 to .010 of an inch
thick
During a rainy weekend back in 1933 Simons was fiddling with an
Oudin coil. This almost forgotten gadget, a close relative of the
Tesla coil, can step up low voltages 1,000 times or more. High
voltage generated in this way has an advantage for the amateur
experimenter in that it is relatively harmless. In the course of
stepping up the voltage the Oudin coil also increases the
frequency of the current, so that it tends to flow through the
skin and away from vital organs such as the heart.
“My original Oudin coil,” Simons recalls, “was part of an
ultraviolet lamp with which I tested mineral specimens for
fluorescence. For no particular reason I decided to replace the
evacuated quartz bulb, which produced the ultraviolet rays, with
an old radio tube of the 01 type. The glass envelope of these
tubes is coated inside with a silvery film of evaporated magnesium
— the so-called “getter” which helps clear the tube of stray
gas during the evacuation process and absorbs any that may be
liberated by the glass walls or metal parts after the seal-off. I
simply held the 01 in my hand and touched its prongs to the
high-voltage terminal of the coil.” Instead of filling with a
lavender glow, like the quartz bulb, the inside of the tube
remained dark but the glass in contact with the magnesium lighted
with a pale greenish fluorescence that reminded me of the glow
emitted by old style X-ray tubes of the gas type. Was the radio
tube producing X-rays?
“To obtain the answer to this question I put a narrow band of
tinfoil around the top of the tube and grounded it — as a
substitute for the electrode previously represented by my hand. I
then fished a small block of wood, which happened to have two
screws in it, from the trash box and placed it on a sheet of
photographic film wrapped in black paper. The combination was
exposed to the energized tube for 15 seconds at a distance of
seven inches. When I had developed the film, I discovered a
wonderful radiograph of the screws — plus a lifetime hobby that
should appeal to anyone interested in physics.”
Why has Simons's hobby failed to catch on? One reason is that
commercial X-ray equipment is costly. Even tubes of relatively low
power are priced at $100 and up. Many other commercial X-ray parts
are also expensive and difficult to procure. The apparatus
supplying high voltage to conventional tubes, while no more
complex than the power supply of a husky radio transmitter, calls
for special rectifying devices, transformers and other components
which are not regularly stocked by dealers in electrical supplies.
Moreover, X-rays have earned a bad reputation as playthings. No
distinction can be drawn between the danger of exposure to a
high-powered X-ray machine and the fallout of an H-bomb. It is a
danger that extends not only to the experimenter but to his
potential progeny. Human evolution is the result of mutations
caused by, among other agents, cosmic rays and the radiations of
radioactive elements in the earth's crust. Any radiation added by
man alters the rate of mutation, and is rightly a cause of deep
concern.
Simons has solved the problem of equipment cost. Protection
against exposure to the rays is not difficult to arrange. With
these two considerations out of the way, X-rays open a range of
experiments equaled by few other phenomena of physics. In addition
to providing a source of X-rays for radiographs, a generator of
X-rays in combination with accessories enables you to measure the
charge of the electron, to study the structure of crystals, to
observe the wave-particle duality of matter and radiation, and to
probe other microcosmic corners.
Like visible light, X-rays are a form of radiant energy. Their
ability to penetrate substances opaque to visible light, however,
is neither unique nor particularly unusual. Many substances opaque
to light are transparent to other electromagnetic waves. For
example, long electrical waves, as well as the shorter ones of
radio, pass freely through dry wood, plaster and other substances
that do not conduct electricity and are opaque to light. If this
were not so, all radio and television receivers would need outdoor
antennas.
On the other hand, a thick sheet of flint glass, which
transmits radio waves and light with no appreciable loss, stops
X-rays. The ability of X-rays to penetrate substances like flesh
and bone is merely their most publicized property. However, this
property provides a striking case of the immediate application of
a scientific discovery. Within weeks of the description of X-rays
by Wilhelm Konrad Röntgen in 1895, surgeons heralded them as a
tool of the first importance.
They are characterized chiefly by extremely short wavelength
— about one ten-thousandth the length of visible light waves.
Like light waves, X-rays can be reflected, refracted, diffracted
and polarized. The techniques by which they are manipulated differ
from those employed with light, just as light techniques differ
from those of radio. The longest X-rays are indistinguishable from
ultraviolet rays; the shortest are identical with gamma rays. The
distinction between the two is largely a matter of definition.
When the emission accompanies the disintegration of a radioactive
substance such as radium, it is called gamma radiation. Identical
waves generated by electronic means are called X-rays.
All radiant energy, including X-rays, has its origin in a
disturbance of electrical charge. Consider a point charge — an
electron — surrounded by a symmetrical electromagnetic field and
moving through space at constant velocity. What happens to the
motion of the field if the central charge is speeded up or slowed
down? Experiments indicate that the field reacts much like a mass
of jelly. When the central charge is accelerated, the disturbance
is communicated radially through the field as a wave motion —
the outside parts of the field requiring an appreciable time
interval to catch up with the center. Work expended in
accelerating the central charge is carried away by the wave as
radiant energy, at a velocity which depends on the nature of the
“jelly.”
In a vacuum the wave attains a maximum velocity of slightly
more than 186,000 miles per second. The length of the wave depends
upon the abruptness with which the central charge is either
disturbed or made to change direction. Violent disturbances
require the investment of more work than gentle ones, and result
in proportionately shorter and more energetic waves. The waves
radiated by electrical power lines, in which a stream of electrons
changes direction only 60 times a second, measure about 3,900
miles from crest to crest.
It is possible to subject electrons to much faster
oscillations. Military radars, for example, are constructed around
magnetron oscillators, small copper chambers that have been called
electrical counterparts of the familiar police whistle. The
cavities are electrically tuned to frequencies on the order of
four billion cycles per second, and streams of electrons forced
into the cavities vibrate at this rate. The resulting
electromagnetic waves measure some three inches from crest to
crest.
As the cavities are made progressively smaller, the pitch goes
up and the wavelength goes down in obedience to the principle that
the smaller the whistle, the shriller its note. Where, then, can a
“whistle” be found that will accelerate charges rapidly or
abruptly enough to create electromagnetic waves a mere 25
thousandths of an inch long — the wavelength of visible light?
Nature provides such systems in the form of molecules and atoms.
The normal, stable atom emits no radiation unless it is acted
upon by an external source of energy. If a fast-moving electron
encounters an atom in its normal state, the interloper may collide
with one of the planetary electrons in the outer orbital shell of
the atom. The impact may cause the electron in the atom to jump to
an orbit still more remote from the center of the atom. A
sufficiently energetic electromagnetic wave impinging on the atom
can accomplish the same end, the requirement being that the
frequency of the wave coincide with the period of the outer
electron's orbital motion.
In either case the atom gains energy from the encounter and
thus becomes unstable. The normal state is soon restored when the
electron hops back into its “home” orbit. This represents the
shifting of a center of charge, and the excess energy is radiated
into space by the accompanying electromagnetic wave. The
abruptness of the jump, and hence the length of the wave, depends
upon the attraction of the positive nucleus for the planetary
electron. When weakly bound electrons occupying an orbit remote
from the nucleus make such jumps, the length of the radiated wave
measures on the order of 25 thousandths of an inch — the
wavelength of light.
This same mechanism accounts for the origin of one kind of
X-ray. When a free electron, accelerated to a velocity of some
18,000 miles per second, collides with an electron occupying an
inner shell of the atom, both the interloper and the innershell
electron may carom into space, as shown in the upper diagram of
Figure 229. The vacancy thus created is immediately filled by an
electron from a shell more remote from the nucleus. The attraction
of the nucleus for this electron is much greater and the jump
accordingly more violent. The resulting wave measures on the order
of 250 millionths of an inch — an X-ray.
This initial jump does not end the display. A vacancy has been
created in the adjacent orbit by the electron that moved inward.
Hence a series of jumps follow as electrons from orbits still more
remote from the nucleus move in to fill the succession of gaps [see
lower diagram, Fig. 229]. In the end the atom must capture a
new electron to complete its outermost orbit. In the meantime the
atom has emitted a series of waves at progressively lower
frequencies, beginning with X-rays and extending through
ultraviolet radiation and visible light to heat.
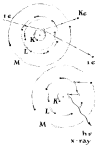
229 Classical representation of X-ray origin in excited atom
The pattern of forces acting between. the nucleus and orbital
electrons responsible for the radiation is unique for each kind of
atom. Measure the radiation emitted at each frequency and you have
an identifying tag for the atom. This is the basis of
spectroscopy. The fundamental particles of the atom move in their
orbits at rates fixed by these same forces. In effect the atom is
“tuned,” much as a group of radio receiving sets might be
pretuned for a group of broadcasting stations.
Atoms low on the periodic table, such as carbon, hydrogen,
oxygen and nitrogen — the stuff of living matter — are far out
of tune with X-rays of extremely high, frequency. When such waves
impinge on protoplasm, they ignore the barrier and sail right
through.
X-rays of the highest frequency, those used for making most
radiographs and for the treatment of disease, are liberated when
the bombarding electron crashes into the massive nucleus of a
target atom. The precise nature of such encounters is not fully
understood, but it appears that when the bombarding electron
collides with the nucleus head-on, and is stopped dead in its
track, the onrushing field may consume the entire mass of the
arrested particle and, in effect, transform the electron into an
X-ray of very high energy and frequency.
Other electrons strike the nucleus glancing blows, and are thus
decelerated. The deceleration is accompanied by the emission of an
X-ray of proportionate wavelength and energy. Hence when a stream
of bombarding electrons plays on a target composed of massive
atoms, the emission of radiant energy includes: (1) X-rays
liberated by the acceleration of planetary electrons,
characteristic of the kind of atoms comprising the target, and (2)
X-rays that span a continuous band of frequencies from the
ultraviolet range to those of almost infinitesimal wavelength.
It was this continuous or “white” X-radiation, arising from
the bombardment of silicon nuclei in the glass of a Crookes tube,
that led Röntgen to his discovery. While studying the green
fluorescence that appears at one stage in the evacuation of the
energized tube, Röntgen observed a bright fluorescence among some
nearby crystals of platinocyanide. The crystals continued to glow
even after he covered the energized tube with black paper. He
concluded that the tube was emitting a previously unobserved form
of radiation.
The Crookes tube consists of a pear-shaped glass bulb that is
partially evacuated and fitted with a cathode at the small end and
an anode at the large end. When a direct-current potential of
about 20,000 volts is connected across the electrodes, positive
ions, accelerated by the electric field, bombard the cathode and
dislodge electrons from the metal. Most of these are attracted to
the anode, but some overshoot the mark. The latter electrons
continue to the end of the tube, where they collide with the glass
target.
Soon after Röntgen's discovery, the mechanism of white X-ray
production was explained by experimental investigation that paved
the way for improvements in the tube. By shaping the cathode in
the form of a paraboloid, for example, the electron stream could
be focused sharply on the metallic target composed of atoms more
massive than silicon. X-rays liberated at the spot were more
energetic and cast sharper shadows than those from the broad
expanse of glass.
Electrostatic machines for the production of accelerating
voltages grew in size until some featured a spinning disk of plate
glass seven feet across, capable of generating 200,000 volts and
currents up to five milliamperes. All the early tubes contained
some gas, the atoms of which were needed as a source of electrons.
The gas imposed an upper limit on the accelerating voltage.
The limitation was removed in 1913 when W. D. Coolidge of the
General Electric Company succeeded in making ductile filaments of
tungsten, which he substituted for the cold cathode of the gas
tubes. With this independent source of electrons, tubes could be
evacuated to the limit of pumping techniques. Accelerating
potentials of 300,000 volts and more became practical. Such power
levels created another problem; the heating of the anode or
target. This problem was first tackled by using tungsten, with its
high melting temperature, at both ends of the tube, then by
cooling the target with water, and finally by focusing the
bombarding electrons in a small spot near the edge of a
motor-driven disk made of tungsten.
Although Simons's tube is not in the class with large X-ray
tubes of the modern Coolidge type, it performs impressively well.
A number of years ago Simons shipped his first machine to George
L. Clark at the University of Illinois who, after testing it,
reported to the journal Radiology: “Simons's apparatus
proves that X-rays can be produced for experimental purposes by a
unit which can be built for a very small fraction of the cost of
an installation of standard commercial equipment. The machine,
when in operation, will produce a beam of X-rays easily detected
for a distance of several feet in all directions. With ‘r’
meter measurements we determined the intensity of the rays to be
three fourths of a Röntgen unit per minute at a distance of three
feet.”
The explanation of this copious radiation, compared with that
of a Crookes tube, appears to lie in the magnesium coating.
Unfortunately the old 01 and tubes of similar construction are
currently in short supply because magnesium is no longer favored
as a material to remove gases, except in certain types of
mercury-vapor rectifying tubes which are unsuitable for X-ray
production. For this reason (plus the fact that he is an
inveterate experimenter) Simons now designs his own tubes and has
them manufactured by a local glass blower. They cost about $15
each. He rarely makes two alike, for the same reason that amateur
telescope makers seldom build two identical instruments. Each
design is a new and exciting experience.
One of Simons's latest designs is illustrated in Figure 230.
This tube is equipped with a disk-shaped cathode of molybdenum and
a magnesium target. It is evacuated to a barometric pressure of
.0001 millimeters of mercury. The over-all length of the tube is
about seven inches. Its emission is substantially greater than
that of the 01 tube. Simons's radiographs, with the exception of
the one showing the screws in a block of wood, were made with it.
Such tubes can be made with a wide variety of target materials and
cathode shapes.
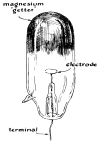
230 X-ray tube designed and constructed by Simons
Almost any source of high voltage can be used for energizing
X-ray tubes, including Van de Graaff electrostatic generators of
the type described in this section. Simons prefers to stick with
Oudin coil. It is easily constructed with hand tools. The job is
simplified if you can lay hands on a vibrator of the type used in
the spark coil of a Model-T Ford. As shown by Roger Hayward's
diagram, Figure 231, and the general view in Figure 232, the
vibrator consists of a core of soft magnetic iron equipped with an
armature of soft magnetic iron and a set of breaker points.
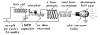
231 Circuit diagram of Simon's X-ray apparatus

232 The source of high voltage for Simon's X-ray machine
The core of the vibrator is wound with 3,800 turns of No. 24
magnet wire and connected in series with the breaker points as
shown. When bridged with the one-microfarad capacitor and
connected to the power line, the self-inductance of the coil is
sufficient to charge the capacitor to a potential of several
hundred volts when the breaker points are adjusted to open at the
peak of the current cycle. The capacitor discharges through the
five-turn primary winding of the Oudin coil. The primary is wound
with five turns of ¼-inch copper tubing on a 2¼-inch plastic
form three inches in diameter.
The secondary winding consists of 5,000 turns of No. 32
enameled magnet wire wound on a ½-inch rod of clear plastic. Each
layer of wire must be carefully insulated with a layer of
varnished cambric that extends well beyond the end of the coil.
When the winding is completed, the secondary coil must be
thoroughly doped with high-grade insulating varnish. Both ends of
the coil are insulated with a tube of varnished cambric.
The assembly is then slipped inside the plastic form on which
the primary was wound. The outside end of the secondary is brought
out through a small hole in the form and soldered to one end of
the primary winding. The inner end of the secondary is threaded
through a four-inch length of ½-inch plastic tubing and soldered
to the inner face of a chromium-plated chair glide, which serves
as the high-voltage terminal. The ends of the primary form are
then closed with disks of ¼-inch plastic and secured in place
with screws at the edge. A ½-inch hole in the center of one disk
admits the tube support for the high-voltage terminal.
The chair glide is lifted temporarily and enough transformer
oil or potting compound poured through the ½-inch tube to fill
the interior. When wired according to the diagram [Fig. 231]
and connected to the power line, the coil will produce some 50,000
to 75,000 volts continuously. The power consumption at 110 volts
and 60 cycles is 35 watts.
As shown in Figure 233, all this apparatus must be housed in a
well-grounded metal container. The X-ray tube must be enclosed in
an inner compartment of lead sheet at least 1/8-inch thick. An
opening in the end of the double housings opposite the tube
provides a window for the X-rays.

233 X-ray machine built by Harry Simons of Kearny, N.J.
WARNING! You must take these precautions
First, Oudin Coils are notorious emitters of radio waves that take
the form of ragged noise. They can black out radio and television
reception for miles around. Federal regulations prohibit the
operation of such devices unless they are thoroughly shielded. If
any stray radiation can be detected on a nearby radio or TV
receiver after the apparatus is assembled as described, it will be
necessary to insert a low-pass filter at the point where the power
cord enters the housing. The design of such filters is available
in standard radio reference texts.
Whenever the machine is in operation, the experimenter must
wear a lead apron and stand well behind the orifice through which
the rays are emitted. Never turn your back to the machine so
that you are between it and the apron! It is also advisable to
place a few exploratory samples of film around the room while the
apparatus is in operation. When developed, these will show the
pattern of radiation and protective lead shielding can be
installed accordingly. Finally, resist the temptation to make
X-ray examinations of the bones in your hands or other body parts.
A frozen fish makes a much safer test object.
The preceding was taken in full from
Section IX. Optics, Heat, and Electronics;
Chapter 3. An Inexpensive X-ray Machine
The Scientific American Book of Projects for The Amateur
Scientist
Library of Congress Card Catalog Number: 60-14286
© Copyright 1960 by C. L. Strong
Disclaimer
How to save and view
schematics
|