Spectra!
If you want to see visible emission line spectra of some elements and light
sources, you are at the right place! (Don's Spectra Site is still under
construction, latest additions 7/4/99.)
Skip to Other Spectra Sites
Please beware that reproduction accuracy is limited. In order to maximize speed
and compatibility with various graphical web browsers, I have largely limited
myself to the 216 colors used by Netscape running under Windows with 8 bit
video. 125 of these colors have nonzero use of all three primaries, about
12-15 are obviously non-spectral magentas and purples, and and a few others
are questionable purplish colors. That leaves only about 75 colors to use,
including bright and dark shades. In addition, the color phosphors used in
computer monitors are not capable of accurately reproducing spectral colors,
especially not deep red, deep green, and deep cyan.
This way to the GIF file alone!
Explanations
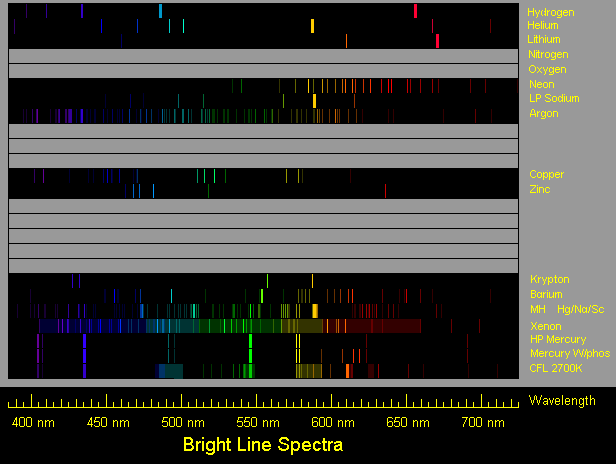
First spectrum is hydrogen, typical of a hydrogen spectrum tube.
Second spectrum is helium, typical of a helium spectrum tube.
Third spectrum is lithium, as typically from a flame or an electric arc.
(Two spaces reserved for future use of nitrogen and oxygen)
Fourth spectrum is neon.
Fifth spectrum is low pressure sodium, but with secondary lines exaggerated.
Sixth spectrum is argon, typical of an argon glow lamp.
(Spaces reserved for future use including iron.)
Next spectrum is copper, drawn using a wavelength table and Ioannis
Galidakis' photos of a copper arc spectrum (see link below). Oxide
lines which may appear in the flame spectrum are not shown.
Next spectrum is zinc, drawn using a wavelength table and a photo by
Ioannis Galidakis of a zinc arc spectrum. Intensity of the red line is
shown for the slightly greenish light blue usual zinc arc, but Ioannis
reports getting a pinkish zinc arc and shows the red line to be brighter.
(Spaces reserved for future use including cadmium.)
Next spectrum is barium. Oxide lines are not included.
Next spectrum is krypton. Ion lines typical of flashlamp use are not included.
Next spectrum is that of the most common variety of metal halide lamp, which is
basically a mercury vapor lamp enhanced with iodides of sodium and scandium.
Next spectrum is that of a xenon flashtube of lower-than-usual pressure,
operated with a higher than usual voltage and a lower than usual energy
level to favor a line spectrum. An actual typical xenon spectrum generally
has a strong continuous spectrum, which I show more dimly than actually
occurs in order to show the lines. The lines are mainly those of excited
xenon ions, rather than excited neutral xenon atoms.
Next spectrum is high pressure mercury vapor, typical of a mercury vapor lamp.
Next one after that is a mercury lamp with the common Deluxe White phosphor.
Next one after that is a compact fluorescent lamp of the 2700K color.
Spectra Photos
by Ioannis Galidakis.
Written by Don Klipstein.
Please read my Copyright and authorship info.
The above spectra artwork is copyright (C) Donald L. Klipstein 1997,
1998, 1999. Distribution of copies mentioning the copyright claim and
authorship is permitted.
Please read my Disclaimer.
